What Is Stress Corrosion Cracking (SCC)Stress corrosion cracking is a method of failure of metal parts. It is usually characterized by severe cracking started by a combination of chemical attack and material stress. It can lead to complete failure of parts without warning and can lead to serious risks. 
History of Stress Corrosion Cracking (SCC)This method of failure was first observed in items made of brass in the warm summers was termed ‘season cracking’. Whilst some brass specimens of failure were observed before 1900, it was during the first world war where brass shell casings were observed to crack when stored near horses that the effect of ammonia ( from the horse urine ) on brass pressings was firmly identified. 
How to reduce the risk of stress corrosion crackingCracking in copper alloys and brasses is generally as a result of internal residual stresses from manufacture adding to any service tensile stress. Stress relief annealing after manufacture reduces these risks. Understanding the effect of chemical attack, particularly from traces of chemicals used in the manufacturing process, will greatly reduce the risk of service failure. Grades with higher resistance stress corrosion crackingGenerally the higher copper content alloys including ‘pure’ coppers are less prone to this type of cracking. Very high stresses of up to 50% of the yield strength and long exposure times are needed to create cracking in ‘pure’ copper materials. Metals most susceptible to SCCBrass alloys with a copper content below 70% are most susceptible to stress corrosion cracking scc. Historically known as Muntz metal, it was in these alloys that stress corrosion cracking scc was first observed. Service failure of pure coppers is extremely rare and limited to cases of very high service stresses. Failure of coppers due to internal stresses alone is unknown. 
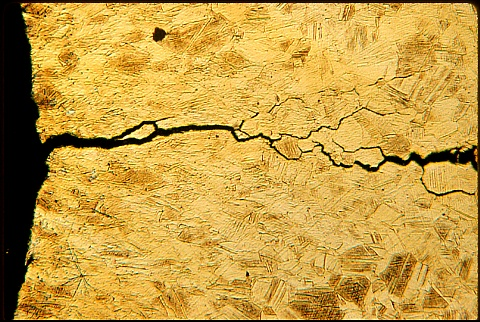
How to repair stress corrosion crackingGenerally it is best to avoid stress corrosion cracking scc by avoiding the types of chemicals that can lead to corrosion cracking. Manufactured parts that exhibit this type of cracking usually cannot be repaired as the risk of further catastrophic failure is too great. How to avoid stress corrosion cracking (SCC)Formed parts should be stress relieved after pressing, in most cases service stresses alone are not high enough to cause cracking. Parts processed with industrial chemicals should be cleaned and washed with water or other unreactive agents. Care should be taken to design parts without crevices that might catch residual chemicals and making washing difficult. Facts in brief about stress corrosion crackingCracking normally occurs between grain boundaries and often displays many branched cracks. Further exposure can cause the remaining metal to become brittle and lead to complete failure. Cracking is normally caused by a combination of residual trace chemicals and internal material stresses. What chemicals cause SCCThe most common chemical exposure for brasses and coppers than can cause stress corrosion cracking is ammonia; a compound found in urine. Some chemical agents such as nitrites can decay into ammonia. Brass has been shown to corrode in aqueous solutions of ammonia, sodium nitrite, citrates, sulfur dioxide and sulfate solutions (Davis JR. Forging and Extrusion. In: Davis JR, ed. Copper and Copper Alloys . Materials Park: ASM International; 2001. p. 220-222.) Stress corrosion cracking of brasses has been shown to occur most severely in ammonia vapour. The amount of ammonia and of copper ions in the form of copper sulphate has been found to increase the corrosion rate of brass. Formation of SCC cracking and effects of the environmentMacroscopically, cracks can occur along geometric discontinuities such as edges of the pressed parts and of any defects or discontinuities within the metal. 
Microscopically, it has been shown that trans-granular and inter-granular cracking of the metal can occur in corrosive environments. The type of microscopic cracking is dependent on the amount and type of corrosion product present, the accessibility of oxidizing agents, or copper ions present, the pH of the environment and the amount of residual stress in the brass products. These factors will either promote diffusion of ionic species into the metal or emphasize the presence of defects within the parts. 
Identifying stress corrosion crackingAny unexpected cracking of formed parts will normally be caused by stress corrosion. In some cases the source of the chemical attack is not obvious. In copper and brass materials environmental factors are not normally the source; ammonia is usually created in an industrial setting. Normal service lifeBy carefully avoiding contamination with the known causes of stress corrosion cracking formed parts of copper and copper alloys can be expected to provide the normal extended service life associated with these alloys. High stresses and the presence of one specific chemical ( ammonia ) are normally required to produce service failure of copper and copper alloys due to stress corrosion cracking. Table 24.4 Stress Corrosion Cracking of Copper Alloys in the AtmosphereTwo Industrial Sites (New Haven, Brooklyn) and One Marine Site (Daytona Beach) [17] CDA | Temper | Time to Failure | Crack Morphologya | New Haven | Brooklyn | Daytona Beach | New Haven | Brooklyn | Daytona Beach | Ident | % Cold Rolled | | 110 | 37% | NFd 8.5 yrs | NF 8.5 yrs | NF 8.8 yrs | – | – | – | | 194 | 37% | NF 8.5 yrs | NF 8.5 yrs | NF 8.8 yrs | – | – | – | | 195 | 90% | NF 3.2 yrs | NF 3.1 yrs | NF 3.1 yrs | – | – | – | | 230 | 40% | NF 8.5 yrs | NF 8.5 yrs | NF 8.8 yrs | – | – | – | | 260 | 50% | NF 35-47 days | 0-23 days | NF 2.7 yrs | I | I | – | | 353 | 50% | 51-136 days | 70-104 days | NF 2.7 yrs | T + (I) | T + (I) | – | | 405 | 50% | NF 2.7 yrs | NTe | NT | – | – | – | | 411 | 50% | NF 2.7 yrs | NT | NT | – | – | – | | 422 | 37% | NF 8.5 yrs | NF 8.5 yrs | NF 8.8 yrs | – | – | – | | 425 | 50% | NF 2.7 yrs | NT | NT | – | – | – | | 443 | 10% | NF 2.7 yrs | NF 2.7 yrs | NF 2.7 yrs | – | – | – | | 40% | 51-95 days | 41-70 days | NF 2.7 yrs | T | T | – | | 40% | 51-67 days | 33-49 days | NF 2.7 yrs | T | T | – | | 510 | 37% | NF 8.5 yrs | NF 8.5 yrs | NF 8.8 yrs | – | – | – | | 521 | 37% | NF 5.7 yrs | NF 5.7 yrs | NF 5.7 yrs | – | – | – | | 619 | 40%, 9% ? phasec | NF 8.5 yrs | NF 8.5 yrs | NF 8.8 yrs | – | – | – | | 40%, 95% ? phase | NF 8.5 yrs | NF 8.5 yrs | NF 8.8 yrs | – | – | – | | 638 | 50% | NF 5.7 yrs | NF 5.7 yrs | NF 5.7 yrs | – | – | – | | 672 | annealed | 0-30 days | 0-134 days | NF 3.1 yrs | I | I | – | | 50% | 0-30 days | 0-22 days | 18-40 days | I | I | I | | 687 | 10% | 517-540 days | 2.3 – NF 2.7 yrs | NF 2.7 yrs | T | T | – | | 40% | 221-495 days | 311-362 days | NF 2.7 yrs | T | T | – | | 40% + orderedb | 216-286 days | 143-297 days | NF 2.7 yrs | T | T | – | | 688 | 10% | NF 2.7 yrs | NF 2.7 yrs | NF 2.7 yrs | – | – | – | | 40% | 4.7-NF 6.4 yrs | 2.7-NF 6.4 yrs | NF 6.4 yrs | T | T | – | | 40% + orderedb | NF 2.7 yrs | NF 2.7 yrs | NF 2.7 yrs | – | – | – | | 706 | 50% | NFb 2.2 yrs | NF 2.3 yrs | NF 2.2 yrs | – | – | – | | 725 | 40% | NF 2.2 yrs | NF 2.3 yrs | NF 2.2 yrs | – | – | – | | 752 | annealed | NF 3.2 yrs | NF 3.1 yrs | NF 3.1 yrs | – | – | – | | 25% | NF 3.2 yrs | NF 3.1 yrs | NF 3.1 yrs | – | – | – | | 50% | NF 3.2 yrs | NF 3.1 yrs | NF 3.1 yrs | – | – | – | | 762 | annealed | 171-NF 3.2 yrs | 672-NF 3.1 yrs | NF 3.1 yrs | T | T | – | | 25% | 142-173 days | 236-282 days | NF 3.1 yrs | T | T | – | | 50% | 142-270 days | 236-282 days | NF 3.1 yrs | T | T | – | | 766 | 38% | 127-966 days | 197-216 days | 754-NF 8.8 yrs | T | T | T | | 770 | annealed | 731-1003 days | 337-515 days | NF 3.1 yrs | T | T | – | | 38% | 137-490 days | 196-518 days | 596-1234 days | T | T | T | | 50% | 153-337 days | 489-540 days | 692-970 days | T | T | T | | 782 | 50% | 23-48 days | 26-216 days | 236-300 days | T + (I) | T + (I) | T |
aI = intergranular, T = transgranular, and parentheses indicates minor mode.
bHeated 400°F, 1/2 hour.
cNormal Structure for this alloy.
dNF = No failures in time specified.
eNT = Not tested Approximate Composition of Alloys| CDA | | Cu | Zn | P | Sn | Pb | Others | | 110 | Electrolytic Tough Pitch Copper | 99.90% | | | | | 0.04% O | | 194 | High Strength Modified Copper | 97.5% | 0.13 | 0.03% | | | 2.4% Fe | | 195 | Strescon | 97.0% | | 0.10% | 0.6% | | 1.5% Fe, 0.80% Co | | 230 | Red Brass 85% | 85% | 15.0% | | | | | | 260 | Cartridge Brass 70% | 70.0% | 30.0% | | | | | | 353 | High Leaded Brass | 62.0% | 36.2% | | | 1.8% | | | 405 | High Conductivity Bronze | 95% | 4% | | 1% | | | | 411 | Lubaloy | 91% | 8.5% | | 0.5% | | | | 422 | Lubronze | 87.5% | 11.4% | | 1.1% | | | | 425 | | 88.5% | 9.5% | | 2.0% | | | | 443 | Arsenical Admiralty Brass | 71.0% | 28.0% | | 1.0% | | 0.05% As | | 510 | Phosphor Bronze 5% | 95% | | 0.1% | 5.0% | | | | 521 | Phosphor Bronze 8% | 92.0% | | 0.1% | 8.0% | | | | 619 | | 86.5% | | | | | 4.0% Fe, 9.5% Al | | 638 | Coronze | 95.0% | | | | | 2.8% Al, 0.40% Co, 1.8% Si | | 672 | | | | | | | | | 687 | Arsenical Aluminium Brass | 77.5% | 20.5% | | | | 2.0% Al, 0.1% As | | 688 | Alcoloy | 73.5% | 22.7% | | | | 3.4% Al, 0.40% Co | | 706 | 90-10 Copper Nickel | 88.7% | | | | | 1.3% Fe, 10.0% Ni | | 725 | Tin Bearing Copper Nickel | 88.2% | | | 2.3% | | 9.5% Ni | | 752 | Nickel Silver, 65-18 | 65.0% | 17.0% | | | | 18.0% Ni | | 762 | | 59.0% | 29.0% | | | | 12.0% Ni | | 766 | | | | | | | | | 770 | Nickel Silver, 55-18 | 55.0% | 27.0% | | | | 18.0% Ni | | 782 | | 65.0% | 25.0% | | | 2.0% | 8.0% Ni, 2% Pb |
|